On November 14, 1985, a groundbreaking discovery was made at Rice University in Houston, Texas, when chemists unveiled a new molecule known as buckminsterfullerene, or more commonly, buckyballs. This molecule, composed of 60 carbon atoms, is celebrated for its remarkable symmetry and has since become a pivotal point in the study of carbon structures.
The journey to this discovery began in the 1970s when Harry Kroto, a chemist at the University of Sussex in the United Kingdom, was investigating the presence of organic molecules within interstellar clouds. These mysterious clouds contained more long carbon chains than astrophysical synthesis theories suggested should exist. This unexpected finding prompted scientists to question the origins of these carbon chains, leading to the hypothesis that cooling red giant stars might be contributing to the interstellar carbon supply.
In September 1985, Kroto collaborated with fellow chemists Richard Smalley and Robert Curl at Rice University. During a ten-day experimental frenzy, they used a laser apparatus to vaporize carbon atoms from a metal disk, allowing them to analyze the resulting molecules within a helium atmosphere. While investigating the expected six- to eight-carbon chains, they stumbled upon a surprise: a stable structure made up of 60 carbon atoms.
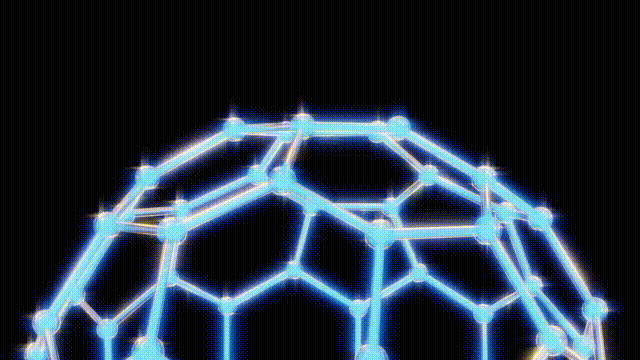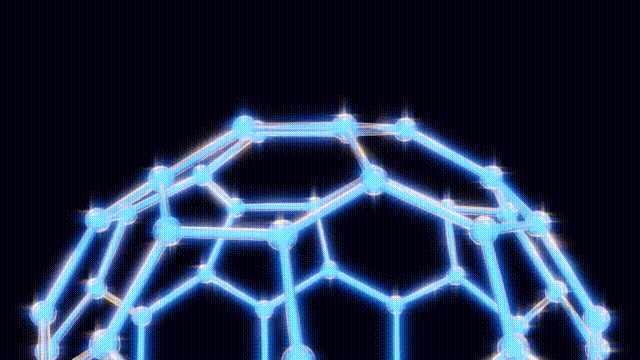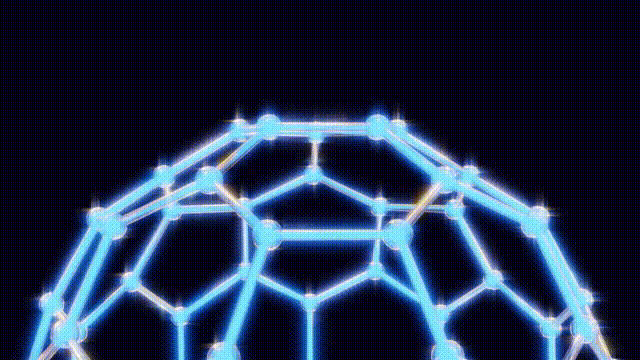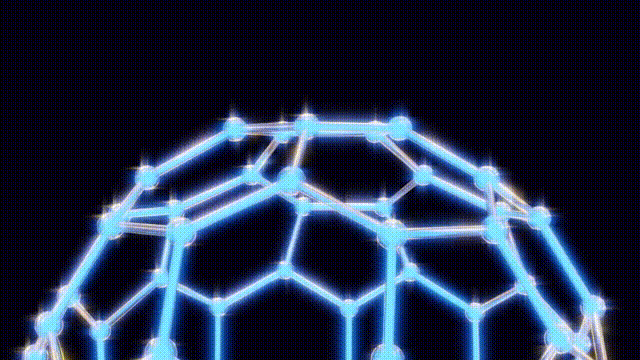
Kroto and his team initially struggled to understand the unusual properties of this new molecule. On September 9, after several days of modeling with everyday materials like toothpicks and jellybeans, they concluded that the molecular structure could not be a flat hexagonal sheet, as previously thought. Instead, they recalled the geodesic dome designs of futurist Buckminster Fuller, which led them to propose a spherical structure for the molecule.
Their findings were formally published in the journal Nature on the significant date of November 14, 1985, marking the official introduction of buckyballs to the scientific community. The name itself pays homage to Fuller, reflecting the molecule’s unique architectural symmetry.
Over the subsequent years, Kroto, Smalley, and Curl continued to explore the properties of fullerenes, a class of closed carbon molecules that includes buckyballs. Their research revealed the potential for fullerenes to be utilized in various applications, from materials science to nanotechnology. By 1990, scientists discovered that an electric arc between carbon rods could produce large quantities of buckyballs, further validating their groundbreaking work.
In recognition of their contributions to chemistry, the trio was awarded the Nobel Prize in Chemistry in 1996. Fullerenes, particularly the related carbon nanotubes, have since demonstrated significant utility due to their strength and exceptional electrical and thermal conductivity. These properties make them valuable in applications such as atomic force microscopy, advanced batteries, and biosensors.
Despite the scientific advancements surrounding buckyballs, their application in mainstream technology remains limited. Researchers continue to explore innovative uses for these molecules, including potential roles in quantum computing and drug delivery systems.
The discovery of buckyballs not only revolutionized the understanding of carbon structures but also paved the way for future innovations in chemistry and materials science, highlighting the enduring significance of this milestone in scientific history.