SynuSight Biotech, ABLi Therapeutics, and XingImaging have formed a strategic collaboration aimed at integrating alpha-synuclein PET imaging into clinical trials for evaluating the efficacy of risvodetinib, a potential disease-modifying therapy for Parkinson’s Disease (PD). This partnership, announced on January 5, 2026, marks a significant advancement in the ongoing battle against neurodegenerative diseases.
As part of the agreement, SynuSight has licensed the PET tracer 18 F-FD4 to ABLi for clinical use. This tracer, developed to visualize alpha-synuclein aggregates in the brain, will be crucial in assessing the therapeutic effects of risvodetinib. SynuSight will receive an upfront payment along with ongoing license-related fees from ABLi, underscoring the financial implications of this collaboration.
XingImaging will manufacture the FD4 tracer at its newly established facility in New Haven, Connecticut. The company will utilize its NeuroEXPLORER, an advanced brain PET imaging system, to enhance the imaging process. This collaboration aims to leverage FD4’s capacity to visualize alpha-synuclein pathology, combining it with ABLi’s innovative blood-borne and tissue biomarkers. Together, these tools will provide a comprehensive approach to evaluating the effects of risvodetinib in clinical settings.
ABLi Therapeutics plans to implement FD4 in two pivotal clinical trials in 2026. The first trial, named ABILITY-PD, will revisit the 120 participants from the Phase 2a 201 Trial completed in the fourth quarter of 2024. This longitudinal study aims to assess the impact of risvodetinib on various biomarkers from the brain, blood, and tissue over a 12-month period. Additionally, it will gather data on motor, non-motor, and dopamine transporter-specific functions, providing vital insights into the relationship between biomarker changes and functional outcomes.
The second trial, known as the c-Abl inhibitor Modification of Parkinson’s Disease (CAMPD) trial, is a Phase 2b/3 study designed to evaluate the efficacy of risvodetinib in a double-blind, placebo-controlled setting with up to 500 participants who have untreated Parkinson’s Disease.
“This collaboration represents a key milestone in the global development of FD4,” stated Roger Fan, CEO of SynuSight. “By integrating molecular imaging technologies with rigorously designed clinical trials, we aim to directly interrogate the disease-modifying potential of risvodetinib at the pathological level.”
ABLi’s Chairman and CEO, Dr. Milton Werner, expressed enthusiasm for this partnership, noting the company’s commitment to expanding its portfolio of disease-related biomarkers. “Adding alpha-synuclein PET imaging to our existing measures enhances our ability to understand the potential disease-modifying effect of risvodetinib in Parkinson’s Disease,” he said.
The significance of this collaboration is further emphasized by the words of Gilles Tamagnan, CEO of XingImaging, who remarked, “This represents a pivotal opportunity to advance one of the most promising alpha-synuclein PET tracers in humans with the potential disease-modifying effect of risvodetinib in PD.”
About Risvodetinib, also referred to as ABLi-148009, it is a selective small-molecule inhibitor targeting non-receptor c-Abl kinases. Designed for once-daily oral administration, risvodetinib aims to halt the progression of Parkinson’s Disease and reverse functional losses associated with it. Currently, no treatments exist that can slow or stop the relentless advancement of the disease. Recently, risvodetinib was the first monotherapy shown to improve quality of life in a randomized, placebo-controlled clinical trial while simultaneously reducing underlying synuclein aggregate pathology.
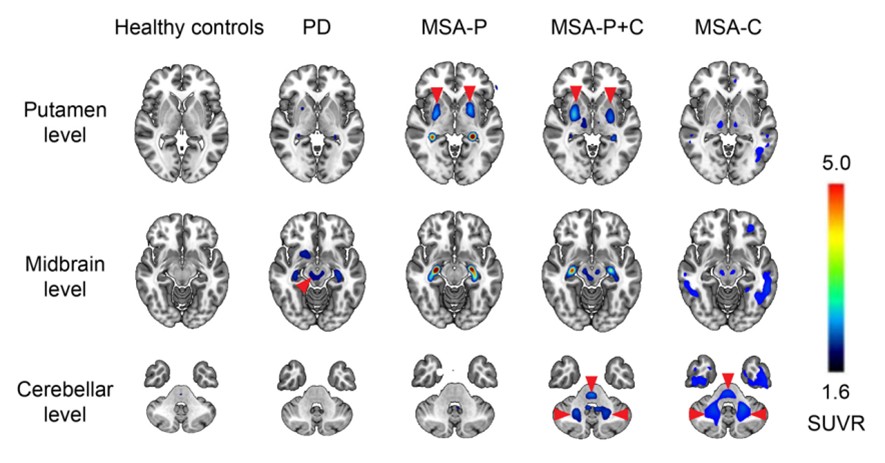
18 F-FD4, developed by SynuSight, is a high-affinity PET tracer designed to image pathogenic alpha-synuclein in Parkinson’s Disease and related disorders. It has shown efficient penetration of the blood-brain barrier and produced high-contrast signals in pathology-relevant brain regions. The tracer received a $3.84 million research grant from the Michael J. Fox Foundation for Parkinson’s Research in 2025, highlighting its potential as a next-generation imaging biomarker.
ABLi Therapeutics specializes in developing small molecule therapeutics targeting diseases associated with the activation or dysfunction of Abelson Tyrosine Kinases. The company focuses on therapeutics for neurodegenerative diseases, particularly those related to Parkinson’s Disease, Multiple System Atrophy, and Dementia with Lewy Bodies.
XingImaging offers comprehensive services across the preclinical and clinical spectrum, emphasizing research using investigational radiotracers. Their work aims to advance clinical neuroscience and nuclear medicine in understanding and treating neurodegenerative disorders.
SynuSight Biotech is dedicated to pioneering diagnostic solutions for neurodegenerative diseases, leveraging advanced technologies to unlock new therapeutic possibilities. The collaboration between these three organizations represents a significant step forward in the quest for effective treatments for Parkinson’s Disease, with the potential to improve the lives of millions affected by this challenging condition.