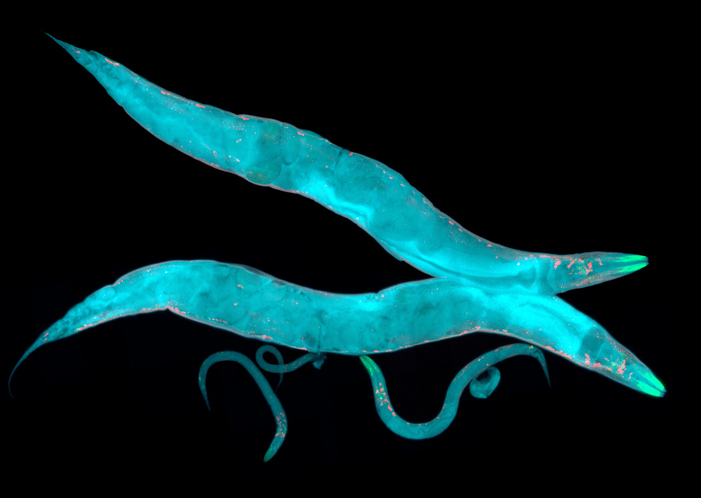
Researchers at UC Davis have revealed significant differences in the structure of cytoplasm within multicellular organisms, using microscopic worms as a model. Their study, published in Science Advances, highlights that the cytoplasm of these worms is more crowded and compartmentalized than that of commonly studied single-celled organisms like yeast or cultured mammalian cells.
The research team tracked the movement of fluorescent particles within the cells of Caenorhabditis elegans, a microscopic nematode known for its transparent skin. This unique feature allowed scientists to observe cellular dynamics in real-time, offering insights into how cellular crowding affects crucial processes such as drug delivery and disease progression. According to first author Xiangyi Ding, a doctoral candidate in the integrative genetics and genomics graduate group, “This changes everything… Crowding in a cell affects any process that depends on molecule movement and interaction.”
Insights from Advanced Imaging Techniques
To explore how particles behave in multicellular environments, the researchers employed Genetically Encoded Multimeric Nanoparticles (GEMs). These particles, approximately 40 nanometers in diameter, were engineered to emit fluorescence, enabling tracking under a microscope at rates reaching 50 frames per second. By inserting DNA instructions for GEMs into the worm’s genome, the team successfully produced thousands of tagged particles in the organism’s intestinal and skin cells.
The findings were striking: the GEMs moved around 50 times more slowly in the worm cells compared to cultured mammalian or yeast cells. Furthermore, the majority of GEMs were confined to specific areas within the cytoplasm, indicating a level of compartmentalization not seen in simpler cell models. Ding remarked, “When we first noticed that the worm cells were constrained, we thought it was a mistake, because this is completely different from what is seen in yeast or mammalian tissue culture cells, which are not constrained.”
Mechanisms Behind Cytoplasmic Organization
The study aimed to identify the factors contributing to this unique cellular organization. The researchers focused on a large protein known as ANC-1, which plays a vital role in maintaining cellular architecture. Disrupting ANC-1 production did not affect the level of crowding but eliminated the confinement of GEMs to specific cytoplasmic regions.
Interestingly, the concentration of ribosomes—cellular structures crucial for protein synthesis—was found to control cytoplasmic crowding. When both ANC-1 and ribosome production were disrupted, GEMs exhibited significantly faster and more unrestricted movement. Co-lead researcher Daniel Starr, PhD, emphasized the importance of these findings, stating, “This shows that cells use two complementary systems to control particle mobility… We already knew that ribosomes were acting like packing peanuts, but until now, we didn’t understand how these two pathways worked together.”
The successful use of GEMs in studying these worms marks a significant advancement, allowing researchers to further investigate other cell types, including neurons. Future studies aim to explore how cytoplasmic dynamics evolve during aging and neurodegeneration, with plans to extend research to more complex organisms such as zebrafish.
This pioneering work emphasizes the necessity of studying cells in their natural environments. Starr noted, “This study highlights the importance of studying cells in living organisms rather than cell culture, because the physical environment of a tissue-cultured cell is so different from anything in an actual organism.” The implications of this research are far-reaching, potentially transforming our understanding of cellular processes in health and disease.