Researchers at the University of Osaka have developed a groundbreaking reagent using the abundant main-group element gallium, which may pave the way for sustainable catalysts in chemical reactions. This innovative compound exhibits transition-metal-like reactivity when exposed to light, marking a significant advancement in the quest for environmentally friendly alternatives to traditional catalysts, which often rely on costly and ecologically harmful transition metals.
Catalysts play a crucial role in facilitating numerous chemical reactions essential for industries such as pharmaceuticals, agriculture, and energy production. Typically, these reactions depend on expensive transition metals like palladium, rhodium, and platinum. Their supply is not only limited but also poses environmental risks due to mining and processing practices.
Gallium’s Unique Properties
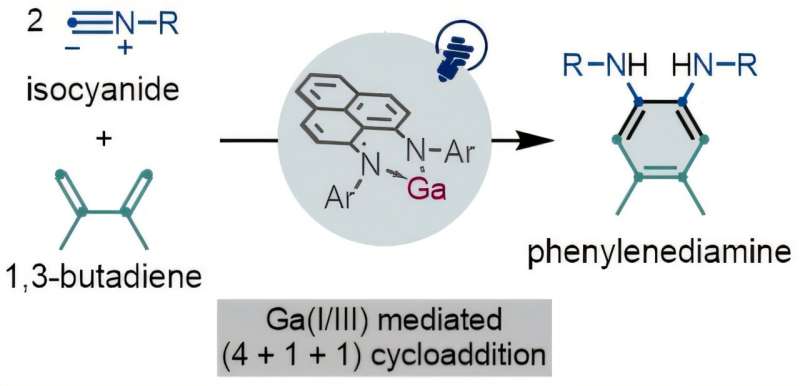
The research team from Osaka has published their findings in the Journal of the American Chemical Society, detailing how their gallium-based reagent can mediate a unique type of redox reaction. This process enables the synthesis of important building-block molecules, specifically phenylenediamines, which are vital for various pharmaceuticals and advanced materials.
Lead author Nijito Mukai stated, “There have been breakthroughs in using heavier group 14 and 15 elements in redox reagents for organic chemistry, but none using group 13 elements. Our redox reagent is the first organic gallium compound to show this type of redox activity.”
The ability of gallium to mimic the reactivity of transition metals under visible light is particularly noteworthy. While traditional catalysts remain unchanged during reactions, the gallium reagent is consumed in the process, providing a new approach to redox reagents from main-group elements.
A Step Toward Sustainable Catalysts
Senior author Takuya Kodama highlighted the significance of this research for the future of sustainable catalysis: “Overcoming the challenges of using a group 13 element will expand the use of main-group elements in redox catalysts.” The findings suggest that utilizing more abundant materials like gallium could alleviate the dependency on rare and environmentally damaging transition metals.
This new strategy offers a promising direction for sustainable chemical processes, emphasizing the potential of earth-abundant elements in reducing the ecological footprint of industrial chemistry. The research not only reflects progress in the field but also serves as a proof-of-concept that could inspire further investigations into the applications of group 13 elements in catalysis.
For more information, refer to the study titled “Synthesis of Phenylenediamines via (4+1+1) Photocycloaddition of 1,3-Dienes and Isocyanides Enabled by a Gallium(I)/(III) Redox: The Key Role of a Phenalenyl-Based Ligand,” published in 2025. The DOI for the article is 10.1021/jacs.5c15802.