Groundbreaking research from the Oregon Health & Science University (OHSU) has led to the creation of fertilizable eggs using human skin cells. This significant development may pave the way for laboratory-grown eggs and sperm aimed at assisting individuals facing infertility or same-sex couples wishing to have genetically related children. However, the study, published in the journal Nature Communications on March 12, 2024, highlights several critical challenges that remain.
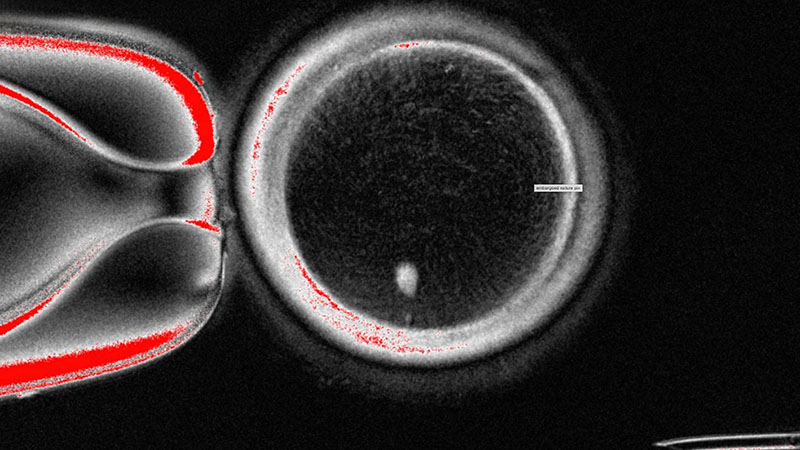
The OHSU team, led by Shoukhrat Mitalipov, removed the nucleus from a human egg cell and replaced it with the nucleus from a skin cell. This innovative approach encountered complications since skin cells contain two sets of chromosomes, while eggs and sperm should each only contain one set. To address this, the researchers induced the egg-like cells to discard the extra chromosomes, subsequently injecting donated sperm to initiate post-fertilization development.
Of the generated cells, approximately 9% survived for six days in laboratory conditions, reaching the blastocyst stage of early embryo development before the experiment was halted. Despite these advancements, the primary concern arose from observed chromosomal abnormalities that hindered the potential of these cells to form genetically normal embryos or eggs.
Shoukhrat Mitalipov, who serves as the director of embryonic cell and gene therapy at OHSU, remarked on the findings as a proof-of-concept, indicating that while the research has made strides, significant improvements are necessary before this technique could be viable for human trials. “We kind of developed this new cell division that can reduce chromosome number,” he stated. “It’s still not good enough to make embryos or eggs genetically normal.”
Reactions from the scientific community have varied. Dietrich Egli, a researcher at Columbia University, expressed concern over the chromosomal irregularities observed. He emphasized the need for caution as these abnormalities could impact the viability of future applications. Conversely, Dr. Eve Feinberg acknowledged the significance of the work, stating, “It seems like this team figured out how to reduce the number [of chromosomes], just not well yet. But it’s an important step and very exciting.”
Despite the challenges, this research represents a promising stride toward developing alternative reproductive technologies. The implications could be profound for individuals struggling with infertility and for couples wishing to conceive children who carry genetic material from both partners. Nevertheless, experts agree that a decade of additional research may be necessary before such techniques can be safely applied in clinical settings.
As this field continues to evolve, the OHSU team’s work offers valuable insights into the complexities of reproductive biology and the potential for innovative solutions to longstanding challenges in human fertility.