Cardiff Oncology, Inc. has released positive data from its ongoing clinical trial, CRDF-004, which evaluates the efficacy of onvansertib in combination with standard-of-care treatments for patients with first-line RAS-mutated metastatic colorectal cancer (mCRC). The trial has demonstrated a confirmed objective response rate (ORR) of 49% in patients receiving a 30mg dose of onvansertib, compared to a 30% ORR in the control group. These findings were reported as of the July 8, 2025 data cut-off.
The preliminary data suggests a favorable trend in progression-free survival (PFS) for the onvansertib group, alongside indications of a dose-dependent response across multiple endpoints, including early tumor shrinkage and depth of response. The trial enrolled a total of 110 patients, with efficacy determined through independent central review of tumor scans.
Dr. Roger Sidhu, Chief Medical Officer of Cardiff Oncology, expressed optimism about the trial results. “We are highly encouraged by the 19% improvement in confirmed ORR and the shorter time to response observed with onvansertib compared to standard treatment alone,” he stated. “The data we are releasing significantly strengthens our earlier findings from December 2024 and supports the potential of onvansertib as a novel therapy for patients with RAS-mutated mCRC.”
Trial Design and Efficacy Data
The CRDF-004 trial was designed to evaluate patients with documented KRAS or NRAS mutations. Participants were randomly assigned to receive either onvansertib combined with standard treatment options—FOLFIRI plus bevacizumab or FOLFOX plus bevacizumab—or standard treatment alone. The primary endpoint measured was ORR, while secondary endpoints included PFS, duration of response, and safety.
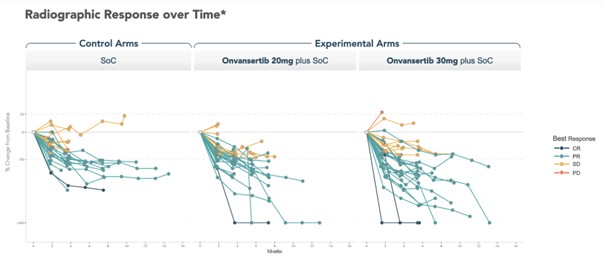
Efficacy data shows that patients on the 30mg dose of onvansertib exhibited deeper responses compared to both the control arm and the 20mg cohort. The use of spider plots to visualize changes in tumor size demonstrated these enhanced responses, indicating a potential benefit of the higher dose.
Preliminary PFS data revealed an early divergence in PFS curves between the onvansertib arms and the control group, although a median PFS has yet to be established.
Safety and Future Directions
The safety profile of onvansertib was assessed in a cohort of 104 patients, with findings indicating that the treatment was generally well-tolerated. No major unexpected toxicities were reported, and grade 3 or higher adverse events were infrequent, with neutropenia being the most common.
Mark Erlander, Chief Executive Officer of Cardiff Oncology, remarked on the significance of the trial’s achievements. “The strength of our data meets the key objectives we set out for this trial and positions us to engage in discussions with the FDA as we advance toward our registrational CRDF-005 trial,” he noted. “We are optimistic about onvansertib’s potential to redefine first-line treatment for RAS-mutated mCRC and plan to provide an update on this program by the first quarter of 2026.”
Cardiff Oncology will host a conference call and live webcast on July 29, 2025, at 4:30 p.m. ET. Interested parties can access the event through the company’s website.
Cardiff Oncology is dedicated to developing innovative therapies through the inhibition of PLK1, targeting various cancers. The company’s lead candidate, onvansertib, is currently under investigation not only for mCRC but also for other malignancies, including metastatic pancreatic ductal adenocarcinoma and triple-negative breast cancer.
For further details, visit https://www.cardiffoncology.com.
This announcement represents a significant step forward in the treatment landscape for patients with RAS-mutated mCRC, with Cardiff Oncology striving to offer advanced therapeutic options.