A groundbreaking technology that manipulates cellular functions through light has been developed by a research team at the Ulsan National Institute of Science and Technology (UNIST). This innovation, known as Mito-AZB, offers the ability to induce reversible cell death using both visible and ultraviolet (UV) light, potentially transforming treatments for superficial cancers such as skin cancer.
Under the guidance of Professor Ryu Ja-Hyoung from the Department of Chemistry at UNIST, the research team created a photoswitchable molecule that can repeatedly assemble and disassemble in response to specific light wavelengths. The findings were published in the journal Nano Letters on November 11, 2025.
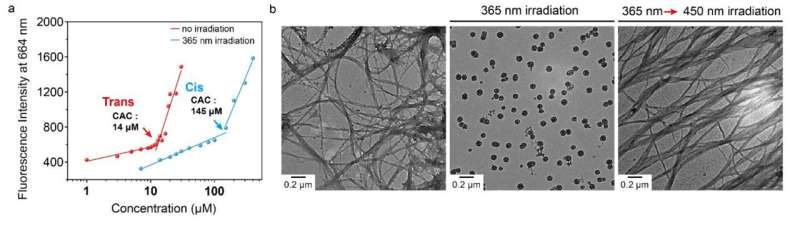
The Mito-AZB molecule is engineered to target mitochondria, the energy-producing organelles in cells. When exposed to visible light at a wavelength of 450 nm, the molecules form a robust fibrous structure that applies mechanical stress to the mitochondrial membranes. In contrast, when subjected to UV light at 350 nm, these fibers disassemble. This back-and-forth process ultimately damages the mitochondrial membranes, leading to the release of pro-apoptotic factors into the cytoplasm and triggering apoptosis, a form of programmed cell death.
Experimental results indicated that the treatment with Mito-AZB, combined with alternating light exposure, caused a collapse of the mitochondrial membrane potential. Moreover, levels of reactive oxygen species and apoptosis-related proteins significantly increased within the cells. Fluorescence microscopy confirmed the molecule’s targeted action by showing accumulation around the mitochondria.
Innovative Design and Versatility
The development of Mito-AZB involved integrating three essential components: a targeting moiety for mitochondrial guidance, an azobenzene unit that undergoes reversible structural changes when illuminated, and a fluorescent dye for real-time visualization. The adaptability of this system was demonstrated when the research team successfully substituted the mitochondrial targeting component with others specific to lysosomes and the endoplasmic reticulum. This flexibility illustrates the potential to selectively disrupt various cellular organelles.
Professor Ryu noted, “This research demonstrates that external light stimuli can precisely manipulate molecular assembly states within cells and modulate cellular responses accordingly.” He emphasized that this technology holds promise for treating superficial cancers through targeted, non-invasive light therapy.
Furthermore, this innovative approach serves as a powerful molecular tool for fundamental research, allowing scientists to transiently inhibit or activate organelle functions. This could significantly advance our understanding of cellular mechanisms, offering new pathways for scientific inquiry and therapeutic development.
For more information on this exciting research, see the original study by Sangpil Kim et al., titled “Photoregulated Assembly–Disassembly Dynamics of Interfering with Organelle Membrane Integrity,” published in Nano Letters. The DOI for the study is 10.1021/acs.nanolett.5c04030.