Researchers at the Weizmann Institute of Science have developed a groundbreaking computational workflow that enables the design of enzymes with catalytic efficiencies exceeding 100,000 M −1 s −1. This advancement brings artificial enzymes closer to the performance levels of natural biocatalysts, eliminating the need for extensive optimization typically required in enzyme development.
The findings, published in the journal Nature, highlight the potential of computational strategies to create proteins capable of catalyzing non-natural reactions with impressive speed and precision. Traditional artificial catalysts have struggled to match the efficiency of their naturally evolved counterparts. This new approach not only challenges that norm but does so with a streamlined process that opens doors for a variety of applications.
Innovative Design Process Yields High-Performance Enzymes
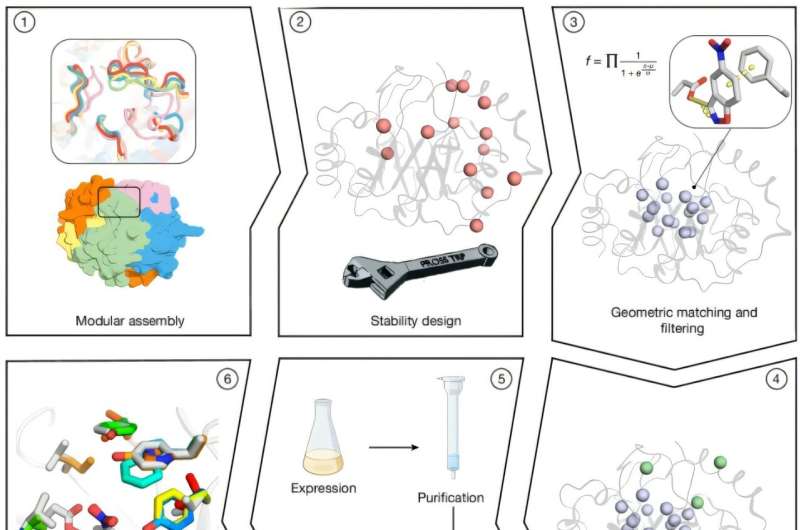
The study, titled “Complete computational design of high-efficiency Kemp elimination enzymes,” showcases a fully computational method to generate stable and active enzymes with minimal refinement. Researchers utilized combinatorial assembly techniques to create thousands of TIM-barrel backbones, a common structural motif found in many enzymes. By merging fragments from homologous proteins, they optimized the orientation of active-site residues to enhance catalytic potential.
To achieve this, scientists employed Rosetta atomistic calculations, allowing them to sift through millions of designs while balancing energy and desolvation factors. From this extensive filtering process, they selected 73 designs for experimental validation. Out of these, 66 were successfully expressed in soluble form, and 14 displayed cooperative thermal denaturation behavior, indicating their stability under varying conditions.
The introduction of 5–8 active-site mutations for each variant resulted in significant improvements in catalytic efficiency. Notably, a variant derived from Des61 (design 61) achieved a catalytic efficiency of 3,600 M −1 s −1 with a turnover rate of 0.85 s −1. Remarkably, eight designs based on Des27 demonstrated catalytic rates between 10–70 times higher than the original, with Des27.7 reaching a kcat/KM of 12,700 M −1 s −1 and a kcat of 2.85 s −1.
Future Implications of Advanced Enzyme Design
The researchers found that a single-point mutation, replacing Phe113 with leucine, elevated catalytic efficiency to an impressive 123,000 M −1 s −1 and a kcat of 30 s −1. These outcomes underscore the importance of active-site preorganization and the enzyme’s ability to adopt various substrate-bound conformations, distinguishing this research from prior models.
Significantly, the most effective variant exhibited remarkable stability, remaining functional at temperatures above 85 °C, while maintaining catalytic efficiencies comparable to those of natural enzymes. The results affirm that current atomistic computational methods are reliable enough to generate efficient enzymes within natural folds without the need for iterative laboratory testing or reliance on artificial intelligence-generated frameworks.
Looking ahead, advancements in enzyme modeling could pave the way for fully programmable biocatalysis, which holds the potential to deliver innovative solutions across various fields, including product manufacturing, therapeutic applications, environmental remediation, green chemistry, and large-scale molecular synthesis. The implications of this research could fundamentally alter how industries approach biocatalysis and synthetic biology in the years to come.
The article was authored by Justin Jackson, edited by Sadie Harley, and fact-checked by Andrew Zinin. The research conducted reflects a significant leap forward in the field of enzyme design, demonstrating the powerful intersection of computational science and biological innovation.