Researchers have discovered that cancer cells can manipulate neighboring healthy cells by transferring mitochondria, the energy-producing components of cells. This groundbreaking study, published in the journal Nature Cancer on August 28, 2023, reveals that tumors are not only able to steal mitochondria from surrounding cells, but can also send their own mitochondria into healthy cells, effectively reprogramming them to support cancer growth.
The lead researcher, Sabine Werner, a biochemist and cell biologist at ETH Zurich, identified a critical protein named MIRO2 that facilitates this transfer. By dispatching their mitochondria, cancer cells can alter the behavior of neighboring fibroblasts, connective tissue cells that play a role in wound healing and tissue repair.
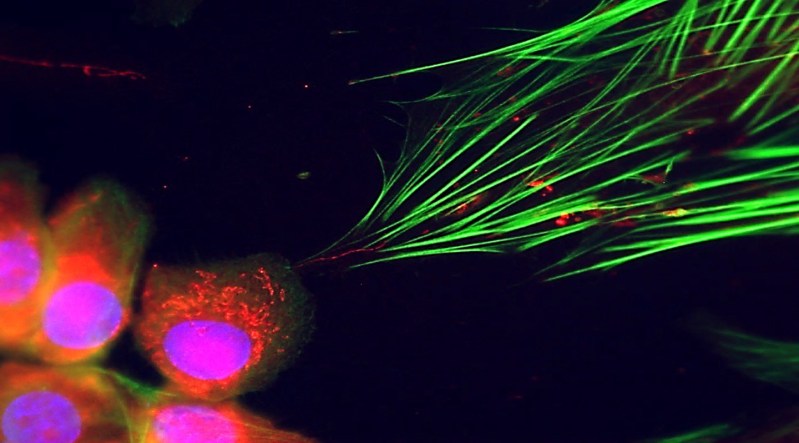
Initially, Werner’s team was investigating how cancer cells communicate with fibroblasts when they stumbled upon an unexpected discovery. They observed long, thin structures connecting the two cell types, resembling tunneling nanotubes, which are known to transport mitochondria and other cellular materials between cells. This observation sparked excitement in the lab, as it indicated a potential mechanism for mitochondrial transfer that had not previously been documented.
In experiments, the team found that when cancer cell mitochondria entered fibroblasts, these recipient cells experienced accelerated growth and increased activity of genes associated with cancer. The altered fibroblasts began to act like “cancer-supporting minions,” contributing to tumor development. When injected alongside cancer cells in mice, these modified fibroblasts prompted tumors to form rapidly, demonstrating the significant impact of mitochondrial transfer on tumor dynamics.
The role of MIRO2 was crucial, as it was identified as the protein that helps transport mitochondria to the edges of cells where the tunneling nanotubes form. Without MIRO2, the transfer of mitochondria from cancer cells to fibroblasts did not occur, highlighting its potential as a therapeutic target in cancer treatment.
This manipulation extends beyond fibroblasts. Other research led by Yosuke Togashi from Okayama University has suggested that cancer cells can also transfer mitochondria to immune cells, diminishing their ability to combat cancer effectively. As understanding of mitochondrial transfer expands, experts like molecular biologist Jiří Neužil from the Czech Academy of Sciences note that the field is rapidly evolving, with many new questions emerging about the underlying mechanisms driving this process.
The implications of these findings are significant as they open avenues for developing targeted therapies aimed at disrupting the communication between cancer cells and their neighbors. Werner emphasizes the importance of exploring unexpected findings in scientific research, as her team’s discovery has highlighted a complex interaction that could play a critical role in cancer progression.
As researchers continue to delve into the intricacies of mitochondrial transfer, the potential for new cancer therapies based on these insights grows. The ability to manipulate cellular behavior through mitochondrial exchange signifies a new frontier in the fight against cancer.