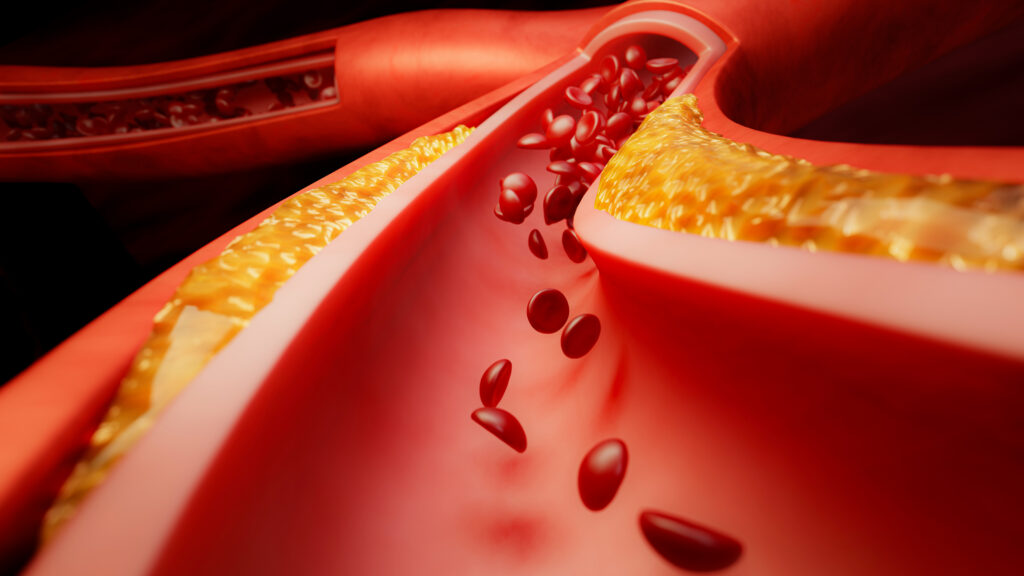
A pivotal trial for a next-generation blood thinner developed by Bristol Myers Squibb and Johnson & Johnson has concluded unsuccessfully. The drug, known as milvexian, failed to meet its primary endpoint in a late-stage trial focused on treating acute coronary syndrome, a condition characterized by sudden reductions in blood flow to the heart.
The announcement, made on October 27, 2023, confirmed that Bristol Myers Squibb would cease the trial after an interim analysis demonstrated that milvexian is unlikely to achieve its intended outcomes. The drug is a selective factor XIa inhibitor, designed to prevent dangerous blood clots that can lead to serious health complications.
Details of the Drug and Trials
Milvexian is currently undergoing testing in three separate Phase 3 trials. These trials aim to evaluate its efficacy in various settings, including cases of acute coronary syndrome, atrial fibrillation, and the prevention of secondary strokes. Acute coronary syndrome encompasses a range of conditions where blood flow to the heart is abruptly compromised, often due to a clot or heart attack.
Despite the setback in the acute coronary syndrome trial, Bristol Myers Squibb remains committed to the development of milvexian. The company plans to continue the remaining trials focused on atrial fibrillation and secondary stroke prevention. These trials will further assess the drug’s potential benefits and risks, aiming to provide a new treatment option for patients at high risk of thromboembolic events.
Implications for the Market and Future Research
The failure of milvexian in this crucial trial could have significant implications for both Bristol Myers Squibb and Johnson & Johnson. Investors often react strongly to trial outcomes, and the unsuccessful result may affect the companies’ stock performance. Analysts had anticipated a robust performance from milvexian, which was seen as a promising addition to the portfolio of anticoagulants.
Moving forward, the companies will focus on gathering data from the ongoing trials. The results from these studies will be critical in determining whether milvexian can still carve out a place in the competitive blood thinner market. The medical community will be closely monitoring these developments, as successful results could offer new hope for patients suffering from conditions related to blood clotting.
The landscape of anticoagulant therapy is constantly evolving, with new treatments being developed to enhance safety and effectiveness. As Bristol Myers Squibb and Johnson & Johnson navigate this setback, the future of milvexian remains uncertain but highlights the challenges inherent in pharmaceutical research and development.